Gambia child deaths: WHO stands by 'dangerous' India cough syrup claim
 WHO
WHOThe WHO has said it stands by its action after India said that four cough syrups linked to child deaths in The Gambia complied with specifications when tested at home.
The WHO had issued an alert in October advising regulators to stop the sale of the syrups, made by an Indian firm.
An Indian government official told the BBC that the WHO was "presumptuous" in blaming the syrups.
But the health body said it was only following its mandate.
"WHO's mandate is to issue global alerts about potential risks. WHO stands by the action taken," an official told the BBC over email.
The health body added that the "contaminated syrups are dangerous and should not be in any medicine, ever".
In late July, medical authorities in The Gambia detected an increase in cases of acute kidney injury among children under the age of five. The government later said around 69 children had died from these injuries.
In October, the WHO said these deaths may be linked to the four cough syrups made by Maiden Pharmaceuticals, an Indian company.
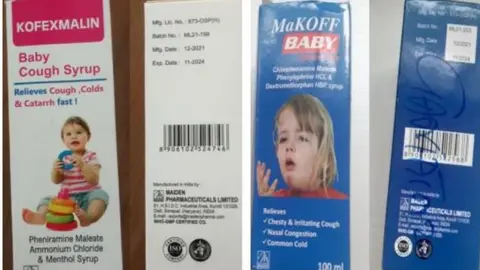
The WHO said it had tested samples of the syrups - Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup - and found that they contained "unacceptable amounts of diethylene glycol and ethylene glycol as contaminants".
Diethylene glycol and ethylene glycol are toxic to humans and could be fatal if consumed.
India then said that it was investigating the products and ordered Maiden Pharmaceuticals to stop production at its main factory in the northern state of Haryana.
On 13 December, Dr VG Somani, India's drugs controller general, wrote a letter to the WHO saying that the samples it tested at a government laboratory "were found not to have been contaminated" with the compounds.
"As per the test reports received from [the] government laboratory, all the control samples of the four products have been found to be complying with specifications," he added.
The test results are being further examined by a panel of Indian experts.
A senior adviser to India's information and broadcasting ministry told the BBC that the WHO had been "presumptuous" in blaming the cough syrups for the deaths of the children.
"Subsequent inspections, tests and studies by Government of India's notified bodies and technical team have shown that WHO's presumptuous statement was untrue and incorrect," said Kanchan Gupta, adding that the health body had "[jumped] the gun without valid scientific reasons".
"When many children die of mysterious sickness, it's a tragedy that means WHO had to act quickly," the agency told the BBC.
"WHO-contracted laboratories in Ghana and Switzerland tested the suspected cough syrups products from The Gambia and confirmed excess levels of ethylene glycol and diethylene glycol," it said, adding that it immediately shared the results with authorities in The Gambia and India, as well as with Maiden Pharmaceutical officials.
In his letter, Dr Somani also said that the panel had requested "specific information" from the WHO on "further details essential to establish the causality" but had not received this yet. The letter did not specify what information the committee had asked for.
When contacted, Dr Somani's office asked the BBC to get in touch with India's health ministry. The BBC has emailed the ministry for comment.
India produces a third of the world's medicines, mostly in the form of generic drugs.
Home to some of the fastest growing pharmaceutical companies, the country is known as the "world's pharmacy" and meets much of the medical needs of African nations.
Dr Somani said in the letter that the WHO's statement, which was "amplified by the global media", had damaged the reputation of the Indian pharmaceutical industry.

Read more India stories from the BBC:
- The rape victim’s mum fighting for India’s daughters
- Is India seeing a surge in grisly copycat murders?
- Shadow of 60-year-old war at India-China flashpoint
- Why is consensual teen sex a crime in India?
- Why Modi continues to be India’s biggest vote-getter
- The Indian women calling themselves ‘proudly single’

