'Nasal tanners left me suffocating in hospital'
 BBC
BBCA woman has told how she was left in hospital "unable to breathe" after suffering a severe reaction to an unlicensed nasal tanning spray she bought online.
Edith Eagle said she felt like she was "suffocating" and "drowning inside her own body" after the allergic collapse she believes was linked to the product.
Nasal tanners are designed to be sprayed into the nostrils and claim to work by administering a substance known as Melanotan II, a chemical that darkens skin pigmentation.
It is illegal to sell medicinal products containing Melanotan II in the UK but as the tanners are sold cosmetically they fall outside that remit.
However, they are not covered by UK cosmetics regulations, meaning they are not subjected to the same scrutiny as other over-the-counter beauty products.
Experts have said they have not been fully researched and could contain toxic ingredients.
 Edith Eagle
Edith EagleMs Eagle said she purchased tanners online believing they would give her a quick and easy bronzed look ahead of a planned holiday to Fuerteventura in April 2023.
They had been recommended to her by someone she knew, but she said she did not realise they were unlicensed and unregulated.
The 47-year-old, from King's Lynn in Norfolk, inhaled the spray twice a day, believing it would give her tan "time to build up before we actually get into the sunshine".
But on the second day of the trip she was rushed to hospital after the apparent allergic reaction spiralled.
"I literally could not breathe," she said.
"And all what went through my mind was, will I even get to the hospital because I could not breathe.
"I can't even explain it, but I was suffocating inside. It was as if I was drowning within my own body."

Ms Eagle said she became suspicious after her stepdaughter, who also used a tanner, spotted a Facebook post from someone who said they had also suffered a bad reaction.
She told her hospital consultant, who asked where she had bought the tanner and what was in it.
He also became suspicious, she said.
"Of course, there was nothing on it apart from a pretty label on the front, no ingredients whatsoever. There was nothing, really, I could show him. And that was a frightening thing," she said.
"Once I was allowed to go back to the hotel the consultant said 'Just remember next time, you may not be so lucky'."
'Side effects'
The products are promoted on social media and are readily available to buy online, while the BBC has also found beauty salons and tanning shops advertising them for sale.
The BBC North West and North East Investigations team visited high street premises to see how easy they were to obtain.
In Manchester and on Merseyside, a reporter managed to purchase several sprays from salons for between £20 and £25, sold with minimal instructions and no acknowledgment of any risks or dangers.

In Newcastle, a staff member at a gym sold a reporter an "extreme strength spray" for £25, with verbal instructions to use it before taking a sun bed.
Also on Merseyside, the BBC was able to buy a "quadruple strength" spray on offer for £20, with the reporter advised to use it morning and night.
Tests at the University of Sunderland found Melanotan II present in varying strengths in six of the 10 samples the BBC had bought.
Stephen Childs, a senior lecturer in pharmaceutical chemistry, said: "There's a massive discrepancy in the amount of this drug in the products people are purchasing.
"The higher the dosage, the more risks that are involved and more side effects are likely to be involved."
Cancer risk fears
He said the samples that did not contain the active ingredient were not necessarily safer as they could include other chemicals that "could be toxic".
"Any unlicensed product really comes with a whole host of dangers. There's no safety data. There are no long-term studies as to the impact on people's health," Mr Childs added.
Another concern is that nasal tanners are often promoted for use in conjunction with sessions on sunbeds to maximise their effectiveness as part of a tanning routine.
Cancer charities believe this could significantly increase the risk of developing skin cancer.

Kerry Rafferty, who started charity Melanoma-Me after her own diagnosis, described the products as "skin cancer in a bottle".
"It's absolutely terrifying, you've got these tanners that nobody really knows what's in them and then they [could be] accelerated by the sunbed use, which we know causes melanoma, so it is a big worry," she said.
"I think that this could be one of the reasons that there is an increase in melanoma at the moment."
Data from North West Cancer Research showed people in the north-west of England were 13% more likely to develop skin cancer than those in the rest of the country.

Chief executive Alastair Richards said the desire for "that tanned look" can often lead people to resort to the excessive use of sunbeds and products like nasal tanners.
He said the true effects of using such products might not be clear for years and could pose an increasing risk of cancer, including among younger people.
"Many of these products are aimed at young people, especially through social media," he said.
"The real danger is that while they might not experience harmful effects now, in the long run they'll be increasing their risk of skin cancer."
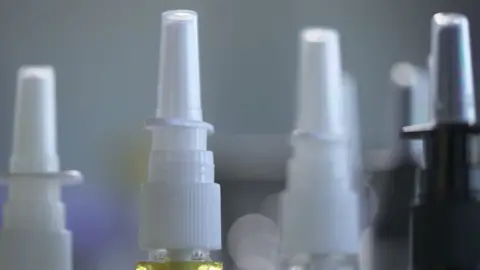
As nasal tanners are not a medicinal product they do not have to be authorised, approved or registered by the MHRA before being sold.
The government's Department for Business and Trade said: "Nasal tanning sprays are not covered by UK cosmetic regulations and must therefore comply with the General Product Safety Regulations 2005.
"This means that anyone selling this product, including online, must ensure they are safe before placing them on the market."
BBC News asked all the stores visited by reporters what steps they had taken to ensure their products met these regulation requirements.
None of them have responded.
Additional reporting by Colette Howe and Jessica Ure
